CMS issued final guidance on the Identification of Selected Drugs for Initial Price Applicability Year 2026
The Department of Health and Human Services Centers for Medicare and Medicaid Services (CMS) recently released initial guidance on Inflation Reduction Act (IRA) implementation of the Medicare Drug Price Negotiation Program for initial price applicability year 2026.
CMS issued final guidance on the Identification of Selected Drugs for Initial Price Applicability Year 2026 (January 1, 2026 to December 31, 2026). An exception to this is the Small Biotech Exception Information Collection Request for which CMS is seeking initial stakeholder feedback prior to issuing a final rule.
The focus of today’s post is exclusive to CMS guidance which has been issued as final. This final guidance applies only to the 10 Medicare Part D drugs which may be subject to selection for negotiation in the initial Price Applicability Year 2026.
So, what does this mean for biopharmaceutical manufacturers?
Medicare drug price negotiations will have both direct and indirect effects on price. For manufacturers with drugs selected for negotiation, the impact will be a direct price reduction. The Congressional Budget Office is estimating that net prices for selected drugs will decrease by approximately 50% on average as a result of negotiation.1 Thus, manufacturers with drugs selected for price negotiation will have a reduced capacity to generate financial returns on investment over the latter years of a brands lifecycle. Late brand lifecycle planning will be further complicated by the fact that manufacturers will not be able to employ strategies such as the launch of an authorized generic or new formulation type as these agents will also be subject to the negotiated price.
For brands which compete in a therapeutic class with a Medicare price-negotiated drug, it is possible that payers may prefer the negotiated drug over its competitors, resulting in a decrease in Medicare access due to an indirect effect. In addition, there may be spillover effects from price negotiation to the commercial line of business as payers seek to leverage the new market price established by the Medicare negotiation program.
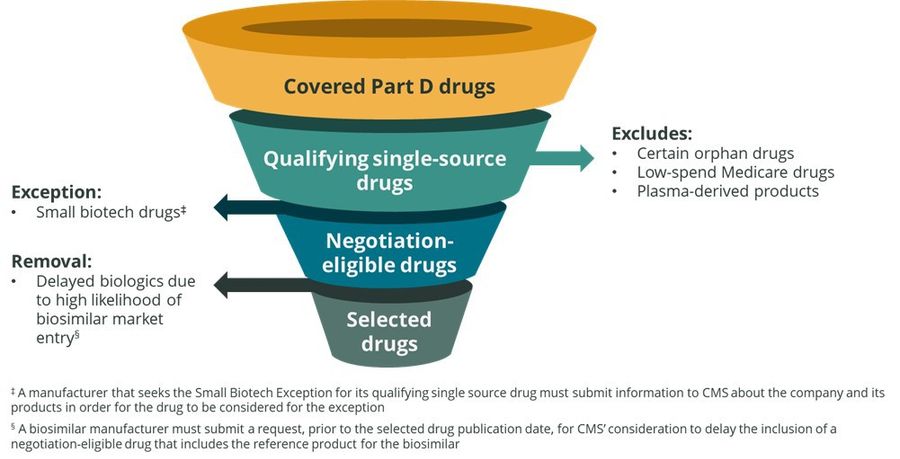
There are several exemptions which preclude a drug from being considered for negotiation and require action on the part of the manufacturer. Manufacturers who believe they may qualify under the “Small Biotech Exception” or for the removal of a biological product due to a high likelihood of biosimilar market entry are required to submit additional information to CMS to determine if their drug may qualify for the exemption:
To receive consideration for the Small Biotech Exception for initial price applicability year 2026, the Submitting Manufacturer must submit the Small Biotech Exception Information Collection Request Form using the CMS Health Plan Management System by the deadline established by CMS (presumed to be June 2023 but will be published on the CMS IRA website in the future).
Biosimilar manufacturers who anticipate that a reference drug for their biosimilar may be selected for initial price applicability year 2026, may submit an Initial Delay Request prior to the selected drug publication date for CMS’ consideration to delay the inclusion of a reference product on the selected drug list. The biosimilar manufacturer eligible to submit the request is the holder of the BLA for the biosimilar or, if the biosimilar has not yet been licensed, the sponsor of the BLA submitted for review by FDA. In addition, the manufacturer would have to assume its biosimilar would be licensed and marketed before September 1, 2025 and believes its request will satisfy the statutory requirements for granting the delay request. E-mail submissions of the initial delay request must be submitted by May 10, 2023.
For more details regarding the final guidance on the Identification of Selected Drugs for Initial Price Applicability Year 2026, please read on.
Additional Details
To identify the Selected Drugs for Initial Price Applicability Year 2026, CMS will execute the following under the final rule:
1. Identify qualifying single source drugs and exclude certain drugs as required under the law.
CMS will define a qualifying single source drug as a covered Part D drug that meets the following criteria:
Drug products
Approved by the FDA
At least 7 years have elapsed since date of approval (initial approval must be on or before September 1, 2016)*
Not approved and marketed under an Abbreviated New Drug Application (ANDA)
Biologics
Licensed under section 351(a) of the Public Health Service Act and marketed in accordance with such licensure
At least 11 years have elapsed since date of licensure (initial approval must be on or before September 1, 2012)*
Is not the reference product for any biological product that is licensed and marketed under section 351(k) of the PHS Act.
CMS will identify a potential qualifying single source drug using:
Drug products will include all dosage forms and strengths of the drug with the same active moiety and the same holder of a New Drug Application (NDA), inclusive of products that are marketed in accordance with different NDAs including, 1) repackages and relabeled products, 2) authorized generics, and 3) multi-market approval (MMA) products imported under the FD&C Act that are marketed in accordance with such NDA(s).
Biological products will include all dosage forms and strengths of the biological product with the same active ingredient and the same holder of a Biologics License Application (BLA), inclusive of products that are marketed in accordance with different BLAs, including, 1) repackages and relabeled products, 2) authorized biologic products and 3) MMA products imported under the FD&C Act that are marketed in accordance to such BLA(s).
CMS will use FDA reference sources, including the Orange Book and Purple Book, to determine whether a generic drug or biosimilar biological product has been approved or licensed for any of the strengths or dosage forms of the potential qualifying single source drugs.
For initial price applicability year 2026, CMS will use the Part D prescription drug event data for dates of service between June 1, 2022 - May 31, 2023 to identify negotiation-eligible drugs based on total expenditures.
CMS will exclude orphan drugs from price negotiation if only one rare disease or indication for which it is the only approved indication for that disease or condition. CMS will use the Orphan Drug Product designation database and approvals on the FDA website to determine if a drug qualifies for the orphan drug exclusion.
CMS will also exclude low spend Medicare drugs with less than $200 million in combined Part D and Part B expenditures.
Finally, plasma derived products, defined as, a licensed biological product that is derived from human whole blood or plasma, as indicated on the approved product labeling also receive an exclusion from being subject to price negotiation. CMS will use product information available on the FDA Approved Blood Products website to identify approved blood products regulated as biological products, the FDA Online Label Repository to verify if the product is derived from human whole blood or plasma and the FDA as consultation when needed.
2. Identify Negotiation-Eligible Part D Drugs for Initial Price Applicability Year 2026
Using the PDE data described above, CMS will calculate the total expenditures under Part D for each qualifying single source drug during the 12-month applicable period.
CMS will remove drugs that are subject to the exception for small biotech drugs. Exceptions to price negotiations for small biotech companies are defined as those with drugs ≤ 1% of total Part D or B expenditures for all covered drugs and accounting for 80% of total expenditures under Part D or B for all covered drugs in 2021 for that particular manufacturer. These exclusions are specific to the time period of 2026 – 2028. CMS is currently requesting public comments on this exception due March 27, 2023.
CMS will then rank the remaining qualifying single source drugs by total expenditures under Part D during the applicable 12-month period.
As a result, CMS will identify 50 qualifying single source drugs that have the highest total expenditures to be considered for price negotiations. The 10 Part D drugs selected for negotiation will be drawn from this list.
3. Selection of Drugs for Negotiation for Initial Price Applicability Year 2026
After removing any biological products that qualify for delayed selection, a “special rule” aimed to encourage biosimilar competition by stipulating that the selection of a biologic for price negotiation could be delayed for up to two years if there is a “high likelihood” of biosimilar competition before a negotiated price would take effect. Other requirements in the special rule specify that the reference drug named in the request will have been licensed for between 12 – 16 years prior to the start of the initial price applicability year on January 1, 2026. Further, the biosimilar manufacturer must not be the same as the reference brand manufacturer and must have not entered into an agreement with the reference brand manufacturer that incentivizes that manufacturer to submit an initial delay request.
CMS will select for negotiation the 10 (or all, if such number is less than 10) highest ranked negotiation-eligible drugs remaining on the ranked list for initial price applicability year 2026.†
A list of the ten Part D drugs selected for negotiation will be published no later than September 1, 2023.
Figure 1: Process for Selecting Drugs for Negotiation for Initial Price Applicability Year 2026
*CMS intends to use the earliest date of approval or licensure of the initial FDA application number assigned to the NDA/BLA holder for the active moiety / active ingredient, or in the case of fixed combination drugs, for the distinct combination of active moieties / active ingredients.
†In the event that two or more negotiation-eligible drugs have the same Total Expenditures to the dollar under Part D and such Total Expenditures are the 10th highest among negotiation-eligible drugs, CMS will rank those negotiation-eligible drugs based on which drug has the earlier approval or licensure date, as applicable, associated with the initial FDA application number for its active moiety(ies) / active ingredient(s), and select based on that ranking until there are 10 selected drugs (or all drugs are selected if the number of negotiation-eligible drugs is less than 10).
1. Congressional Budget Office. How CBO Estimated the Budgetary Impact of Key Prescription Drug Provisions in the 2022 Reconciliation Act. (February 2023).
Questions or inquiries on the IRA or another Medicare business question? Please contact us at [email protected].