FDA Offers New Draft Guidance on Considerations for Rescinding Breakthrough Therapy Designation
The FDA recently released its draft guidance for industry on “Considerations for Rescinding Breakthrough Therapy Designation”.
The FDA recently released its draft guidance for industry on “Considerations for Rescinding Breakthrough Therapy Designation”. Breakthrough therapy designation (BTD) is one of four distinct mechanisms to speed the development and availability of drugs treating serious or life-threatening conditions as seen in Figure 1.
Figure 1. FDA-Expedited Program Summary

While they all aim to improve FDA efficiency, these programs differ in their timing, requirements, and overall approach to the drug development and approval process.
Table 1. Summary of Key Requirements and Features of FDA-Expedited Programs
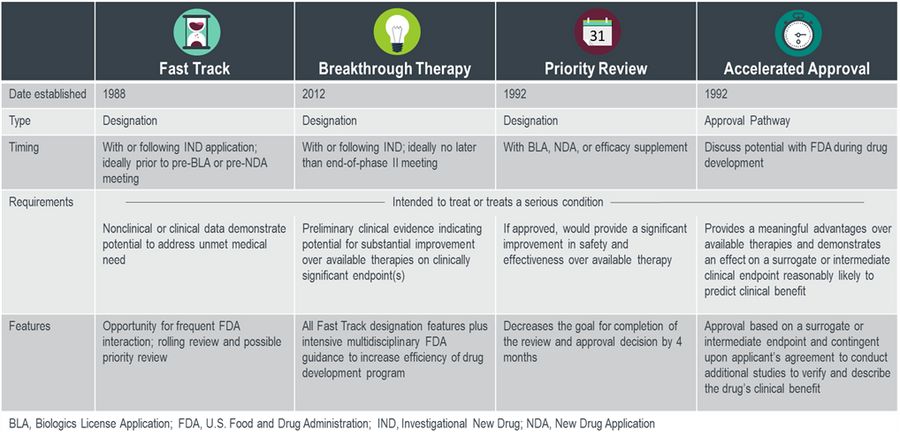
Source: Kluetz P and Donoghue M. ASCO Post (2014). https://www.ascopost.com/issues/february-15-2014/fda-programs-to-expedite-drug-and-biologic-product-development/
Established in 2012, the breakthrough therapy designation was designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug provides substantial improvement over existing therapies.
The recent draft guidance describes how, during FDA evaluation of a drug development program, they may consider whether to rescind a BTD.
According to the draft guidance, “the information supporting the granting BTD for a particular drug may change over time. Some drugs that appear promising in early development may not be shown to be safe or effective in later trials, or the magnitude of a treatment effect suggested by early development may not be observed in later stages of development. Accordingly, given the resource-intensive nature of the BTD program, and in keeping with the Agency’s authority to grant BTD only to drugs that meet the legal criteria, FDA periodically assesses whether designated products continue to meet the criteria for BTD. If the designation is no longer supported by subsequent data, FDA may rescind the designation.”
The guidance offers three reasons of when a BTD can be rescinded:
A different drug is approved to treat the unmet need that informed the rationale for granting BTD. As a result of this new therapy, the BTD drug no longer meets the BTD criteria regarding substantial improvement over existing available therapies. Note that another drug approved under accelerated approval generally will not be considered sufficient to lead to rescinding BTD.
Emerging data for the designated drug no longer support a finding that “preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies.”
The designated drug’s sponsor is no longer pursuing the drug’s development program for the use that was the basis for BTD.
As of December 2021, the FDA notes there have been a total of 1,020 breakthrough therapy requests received and 417 BTDs granted (a 41% initial approval rate). In that same time period, the FDA has rescinded 35 BTDs, with more than half being rescinded between 2019 and 2021.
Figure 2. Breakthrough Therapy Designations Withdrawn after Granting or Rescinded by Fiscal Year (July 9, 2012 - September 30, 2021)
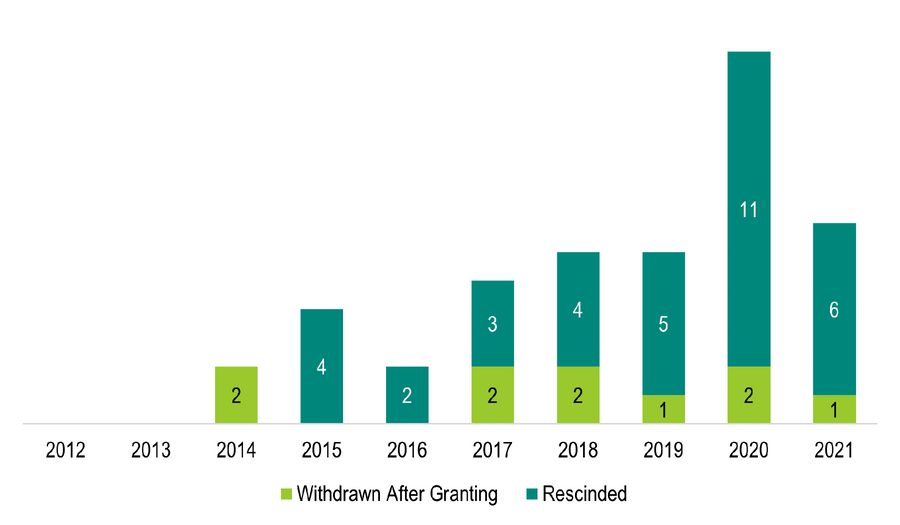
Source: FDA. https://www.fda.gov/media/95269/download
When finalized, the guidance will represent the FDA's current thinking on this topic.
Implications
For manufacturers, the draft FDA guidance suggests that a comprehensive post-launch strategy will be required to fulfill the promise of a BTD. From monitoring the competitive environment for drugs with the potential for approval of the previously unmet need, to a carefully planned post BTD clinical program which has the potential to support the preliminary clinical evidence, it is critical that manufacturers allocate the needed resources to achieve the brand promise inherent in a BTD.