A Snapshot of the Conclusions on Long-term Value of Healthcare Treatments Conducted by the Institute for Clinical and Economic
Institute for Clinical and Economic Review has published multiple assessments of healthcare technologies which provide a volume of evidence to better understand the quantitative conclusions by each evaluation relative to all evaluations undertaken to date. This inference provides a focused evidence of the evaluated therapies and disease areas with the aim of better understand modeling methods, approaches, analyses and conclusions.
Background/Methods
The Institute for Clinical and Economic Review, a non-profit institute evaluates the cost-effectiveness of drugs, medical devices, medical procedures, and diagnostics. The Institute for Clinical and Economic Review has published multiple assessments of healthcare technologies which provide a volume of evidence to better understand the quantitative conclusions by each evaluation relative to all evaluations undertaken to date.
BluePath conducted a focused evidence of the evaluated therapies and disease areas with the aim of better understand modeling methods, approaches, analyses and conclusions. The focus below is a summary of the findings as they relate to the long-term value for money analysis with the primary outcome of the incremental cost-effectiveness ratio (ICER, defined as the incremental cost per QALY gained).
Key Findings
A total of 39 final evidence reports have been published as of October 2017. Of those reported, 19 disease areas were evaluated including, but not limited to oncology, autoimmune diseases, cardiovascular disease, and diabetes. A total of 80 medications were analyzed with 38 data extraction points covering methods, analyses and results.
As shown in Figure 1, 28% of medications evaluated were below a WTP of $150,000/QALY. About 14% fell below the $100,000/QALY WTP threshold.
Figure 1: Distribution of Incremental Cost-effectiveness Ratios by Disease State
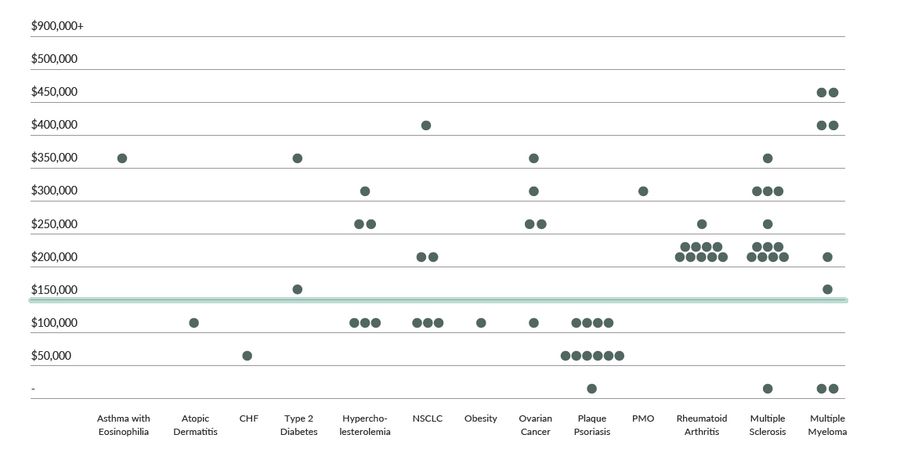
The findings suggest wide variability in ICER values, with some clustering within disease states, specifically plaque psoriasis and rheumatoid arthritis. In addition, value-based pricing analyses using $150,000 per QALY gained recommended discounts ranging from an average of ~5% for therapies evaluated in type 2 diabetes to up to 93% for therapies evaluated in the treatment of Non-alcoholic Steatohepatitis (NASH). Only one therapy for CHF was viewed as under priced and a price increase was needed to achieve the willingness to pay threshold.
Conclusion/Action Steps
The Institute for Clinical and Economic Review continues to generate reports with more recent evaluations, such as within the CAR-T space producing favorable ICERs. This report will be updated regularly as the findings also document the evolution of the reports over time with significant gains observed in 2017 relative to earlier years. These findings are of interest in understanding macro trends; however, the results reflect more granular issues surrounding model design and assumptions made by the Institute for Clinical and Economic Review, which very significantly across relevant stakeholders.
It is imperative base case results better incorporate robust and meaningful sensitivity and scenario analysis that consider real world evidence and effectiveness factors that best reflect the intent of a cost-effectiveness model (versus cost-efficacy), which is to estimate the long-term value when used in the real-world.